Introduction
Vitamin D deficiency is a modifiable risk factor for multiple malignancies. There is growing evidence that associates vitamin D deficiency with progression-free survival (PFS) and overall survival (OS) in patients with classic Hodgkin lymphoma (cHL). Supplemental ergocalciferol/cholecalciferol may improve chemosensitivity of malignant cells to chemotherapy as evidenced by reduction in the rate of tumor growth in a cHL- xenograft animal model. The goal of our study is to explore the association of pretreatment vitamin D levels on survival outcomes of patients with cHL.
Methods
We retrospectively reviewed the records of patients who were first seen at The University of Texas MD Anderson Cancer Center between January, 2016 and May, 2020 for newly diagnosed cHL. Patient charts were reviewed to assess demographic information, clinical staging at the time of vitamin D assessment, pretreatment 25-hydroxyvitamin D (25(OH) D) level and vitamin D supplementation. Vitamin D deficiency was defined as a 25(OH)D level < 30 nmol/L. PFS and OS outcomes were evaluated for these patients.
Descriptive statistics including mean, standard deviation, median, and range for continuous variables such as age, and 25(OH) D level, and frequency counts and percentages for categorical variables such as race, gender, vitamin D supplementation and response were analyzed. The Kaplan-Meier method was used for time-to-event analysis including PFS and OS. Median time to event in months with 95% confidence interval (CI) was calculated. The Log-rank test was used to evaluate the difference in time-to-event endpoints between patient groups. Statistical software SAS 9.4 (SAS, Cary, NC) and S-Plus 8.2 (TIBCO Software Inc., Palo Alto, CA) were used for statistical analyses.
Results
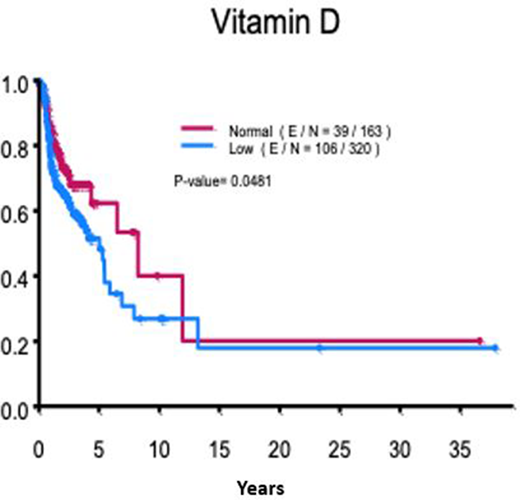
644 patients met the inclusion criteria of which 483 patients had their vitamin D levels assessed at the time of initial visit to this center. The median patient age at diagnosis was 33 years with 52% males. Advanced stage (stages III and IV) occurred in 45% of patients of which the International Prognostic Score was ≥4 in 13% of patients. Patients received doxorubicin, bleomycin, vinblastine and dacarbazine (ABVD) based therapy (77%), brentuximab vedotin (BV) based therapy (13%) and other agentss (6%). Patient demographics are outlined in Table.1. Pretreatment 25(OH)D level was assessed in 75% of the patients. The median 25(OH)D level was 25 nmol/L (range: 2-78 nmol/L). Vitamin D deficiency was present in 320 of 483 (66%) patients. Ergocalciferol/cholecalciferol supplementation was used in 29% of patients. There was no statistically significant association of vitamin D deficiency with advanced stage (p-value: 0.64). PFS rate at 10 years was significantly longer in patients with normal 25(OH)D level (40% vs 27%, p-value: 0.0481). Ergocalciferol/cholecalciferol supplementation was associated with a 6% improvement of PFS, however this difference was not statistically significant. No OS difference was noted between vitamin D deficient and non-deficient patients, an observation that persisted in patients on vitamin D supplementation versus not on supplementation.
Conclusions
Vitamin D deficiency was associated with inferior PFS with a 13% difference in vitamin D deficient versus non-deficient patients. There was a numerical PFS benefit associated with ergocalciferol/cholecalciferol supplementation. An OS benefit was not observed as the duration of follow up may not have been sufficient to observe the differential impact of vitamin D levels. Vitamin D screening and replacement is done in patients with newly diagnosed cHL and should be encouraged given the potential benefit from such approach. Prospective studies are warranted to establish the relationship between vitamin D level, supplementation and outcomes in cHL patients.
Parmar:Cellenkos Inc.:Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding.Nieto:Affimed:Consultancy, Other: Grant Support;Novartis:Other: Grant Support;Astra Zeneca:Other: Grant Support;Secura Bio:Other: Grant Support.Chuang:Sage-Evidence=Based Medicine & Practice:Consultancy.Wang:Lu Daopei Medical Group:Honoraria;Beijing Medical Award Foundation:Honoraria;OncLive:Honoraria;Molecular Templates:Research Funding;Verastem:Research Funding;Dava Oncology:Honoraria;Guidepoint Global:Consultancy;Nobel Insights:Consultancy;Oncternal:Consultancy, Research Funding;InnoCare:Consultancy;Acerta Pharma:Research Funding;VelosBio:Research Funding;BioInvent:Research Funding;Juno:Consultancy, Research Funding;Kite Pharma:Consultancy, Other: Travel, accommodation, expenses, Research Funding;Pulse Biosciences:Consultancy;Loxo Oncology:Consultancy, Research Funding;Targeted Oncology:Honoraria;OMI:Honoraria, Other: Travel, accommodation, expenses;Celgene:Consultancy, Other: Travel, accommodation, expenses, Research Funding;AstraZeneca:Consultancy, Honoraria, Other: Travel, accommodation, expenses, Research Funding;Janssen:Consultancy, Honoraria, Other: Travel, accommodation, expenses, Research Funding;MoreHealth:Consultancy;Pharmacyclics:Consultancy, Honoraria, Other: Travel, accommodation, expenses, Research Funding.Lee:Takeda:Research Funding;Seattle Genetics:Research Funding;Oncternal Therapeutics:Research Funding;Guidepoint Blogal:Consultancy;Celgene:Research Funding;Bristol-Myers Squibb:Consultancy, Research Funding;Aptitude Health:Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal